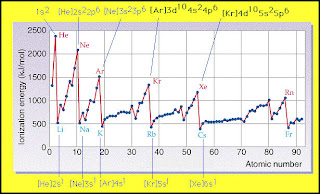
Ionization
-Ionization energy means the energy needed to remove electrons from an atom. Large atoms require low ionization energy while small atoms require high ionization energy. This quantity was formerly called ionization potential, and was at one stage measured in volts
Atomic Radius
-The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius.
Electro negativity:
-Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself and thus the tendency to form negative ions
-------------------------------------------------------------------


没有评论:
发表评论