- n is the # of energy level
-the energy different between 2 particular energy level is called quantum of energy
-Orbit is the actual region of space occupied by an electron in a particular energy level
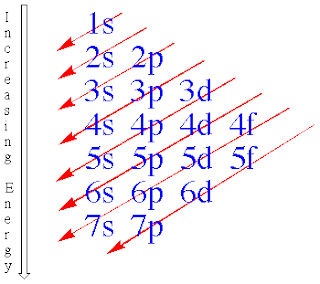
- n=1:only the s-type is possible
- n=2:the s and p types are possible
- n=3:the s p and d types are possible
- n=4: the s p d f types are possible
-----------------------------------
- s type sub shell consists of 1 s-orbital
- p type sub shell consists of 3 p-orbital
- d type sub shell consists of 5 d-orbital
- f type sub shell consists of 7 f-orbital
-2 Electron can be placed in each orbit
-a shell is the set of all orbitals having the same N- value
E.g the third shell consists of the 3s 3p 3d orbitals
-a subshell is a set of orbitals of the same type
- An electron configuration is a description of which orbitals in an stom electron configuration
E.g Ne(1s2 2s2 2p6)
=---------------------------------------------
Core Notation
-the set of electrons belonging to a given atom can be divided into 2 subsets: the core electrons and th outer electrons
-CORE: the set of electrons with the configuration of the nearest noble gas having an atomic number Less than that of the atom being considered
E.g Al(1s2 2s2 2p6 3s2 3p1)------>Al([Ne] 3s2 3p1 )
CORE OUTER
EXCEPTION:
Cr([Ar]4s2 3d4)---->Cr([Ar]4s1 3d5) --to make 2 half filled sub shells
Cu([Ar]4s2 3d9)---->Cr([Ar]4s1 3d10)--to make a hall filled sub shell and a filled sub shell
-----------------------------------------------------
没有评论:
发表评论