Rules:
-The nuclear is represented by the atomic symbol
-For individual element determine the number of valence electrons
-Each orbit get 1e before they pair up
-Each bond represent 2e
-All Valence electrons must be used
--------------------------------------------------------------
Organic Compound
Single bonded:
C2H6
C:4e- H:1e-
---------------------------------------------------------
Double bonded
CO2 .. .. .. ..
C:4- :O::C::O: ---------:O=C=O:
O:6-
-------------------------------------------------------------
Triple bonded
N2
N:5e- ...... .._..
N::N -------- N=N
----------------------------------------------------------
Lewis Structure
-H F are never at the center
-place metal in the center if possible
-single atom in the center
-atom needs most e-in the center
e.g .. ..
N2H2 H:N::N:H H-N=N-H
------------------------------------------------------
Polyatomic
NH4 + H + H
.. |
[ H: N :H ] H-N-H
.. |
H H
-----------------------------------------------------------
<iframe width="425" height="349" src="http://www.youtube.com/embed/QKoA3fZ29B0" frameborder="0" allowfullscreen></iframe>
2011年6月6日星期一
Chemical Bonding
1.Ionic
2.Non-polar Covalent
3.Polar Covalent
- If the electrons are shared equally -A non polar covalent is formed
shared unequally-A polar covalent is formed
transfered between 2 atoms-Ionic is formed
----------------------------------------------------------------------------
1.Ionic Bond
-All boding is based on the electrostatic relationships
-Opposite charges attract each other
-Like charges rebel each other
-Electrostatic force is a force that exist between charged particles as a result of attraction of repulsion
-Atom with high electro negativity values have high ionization energy because they strongly attract their elections and difficult to remove
--------------------------------------------------------------------------------
2.Covalent Bonding
-Intra molecular covalent bonds are hold together by intermolecular forces to form covalent
----------------------------------------------------------------------------------
2.Non-polar Covalent
3.Polar Covalent
- If the electrons are shared equally -A non polar covalent is formed
shared unequally-A polar covalent is formed
transfered between 2 atoms-Ionic is formed
----------------------------------------------------------------------------
1.Ionic Bond
-All boding is based on the electrostatic relationships
-Opposite charges attract each other
-Like charges rebel each other
-Electrostatic force is a force that exist between charged particles as a result of attraction of repulsion
-Atom with high electro negativity values have high ionization energy because they strongly attract their elections and difficult to remove
--------------------------------------------------------------------------------
2.Covalent Bonding
-Intra molecular covalent bonds are hold together by intermolecular forces to form covalent
----------------------------------------------------------------------------------
Polarity
-If there is an imbalance with electrical charge then a molecule is polar
-If the electrical charge is the same---is non polar covalent
-higher electronegativity will form a partial negative charge δ-(0<=> -1)
-lower electronegetivity will form a partial positive charge δ+(0<=>1)
-An arrow sigh is usually used to indicate the migration of electrons towards the move electronegative atom.
E.g.
δ+H------Fδ-
+------->
---------------------
H:2.20
-Cl:3.16
-Cl:3.16
=0.96
δ+H------Clδ-
----------------------------------------------------------------------------------
2011年5月16日星期一
Periodic table trend
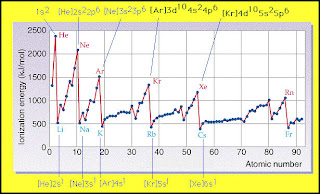
Ionization
-Ionization energy means the energy needed to remove electrons from an atom. Large atoms require low ionization energy while small atoms require high ionization energy. This quantity was formerly called ionization potential, and was at one stage measured in volts
Atomic Radius
-The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius.
Electro negativity:
-Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself and thus the tendency to form negative ions
-------------------------------------------------------------------
Periodic table history and Family of periodic table
Periodic table history
-A necessary prerequisite to the construction of
the periodic table was the discovery of the individual elements.
-By 1869, a total of 63 elements had been discovered.
--------------------------------------------
-Between 1863 and 1866 John Newlands
showed that by assigning Hydrogen an
arbitrary mass of 1 and ordering the known
elements by their masses, every eighth
element shared a common set of properties
-He called this the “law of octaves
----------------------------------------------
-1869, Dimitri Mendeleev published a method of
organizing the elements according to both
their masses and their properties.
-Mendeleev showed that when the elements
are listed according to masses, certain
properties recur PERIODICALLY.
- He broke the list into a series of rows
(PERIOD) and columns (GROUP).
-----------------------------------------------
l He placed elements in certain groups based on
their properties in spite of contrary indications by its
mass
Mendeleev left gaps in his table for elements, which
he proposed had yet to be discovered.
He was able to predict the properties and
characteristics of the undiscovered elements so
accurately that when they were discovered the
predicted value was quite exact.
The periodic table allowed chemists to organize
and understand their data and predict new
----------------MODERN PERIODIC TABLE-----------------------------
Is organized according to atomic number rather than
atomic mass. (This solved the problems where
different isotopic abundances caused the masses to
be “out of order”. Example: Ar and K, Co and Ni)
The periodic law summarizes the periodic table.
l The Periodic Law: Properties of the chemical elements
recur periodically when the elements are arranged from
lowest to highest atomic numbers
Major Divisions in the Periodic Table
l Period: The set of all elements in a given row going
across the table.
l Group or Family: The set of all elements in a given
column going down the table.
-------------------------------------------------------
THE Family of Periodic table
----Alkali Metal = elements in the first column (Except H)
----Alkaline Earth Metals = The elements in the second column
----Halogens = second column from the end on the right hand side. Starting with Fluorine.
----Noble Gases = Far right side of the table. Starting with helium.
----Lanthanide = elements in the first row shown underneath the table. Starting with lanthanum.
----Actinides = underneath the Lanthanides. Starting with actinium.
..................................................
Metals, Nonmetals, Semiconductors
Metal: reflect light when polished
are opaque
are good conductors of electricity and heat
are malleable or rolled into thin sheets and ductile
are usually solid at room temperatures
..........................................................
Non metals
Non metal: liquids or brittle solids at room temperature
Poor conductors of heat and electricity
Solids are dull to lustrous in appearance and opaque to translucent
Non – Metals can be divided into two subgroups:
1.Very low electrical conductivities
2.Fair to moderate conductivities
.........................................................
Semiconductor
A non-metal having an electrical conductivity, which increases with teperature.
Semiconductors (Metalloids or Semimetals) have properties which resemble metals more than nonmetals
Important difference is that metal conductivity
decreases with increasing temperature whereas the electrical conductivity of semiconductors
increases with increasing temperature.
0.................................................
-A necessary prerequisite to the construction of
the periodic table was the discovery of the individual elements.
-By 1869, a total of 63 elements had been discovered.
--------------------------------------------
-Between 1863 and 1866 John Newlands
showed that by assigning Hydrogen an
arbitrary mass of 1 and ordering the known
elements by their masses, every eighth
element shared a common set of properties
-He called this the “law of octaves
----------------------------------------------
-1869, Dimitri Mendeleev published a method of
organizing the elements according to both
their masses and their properties.
-Mendeleev showed that when the elements
are listed according to masses, certain
properties recur PERIODICALLY.
- He broke the list into a series of rows
(PERIOD) and columns (GROUP).
-----------------------------------------------
l He placed elements in certain groups based on
their properties in spite of contrary indications by its
mass
Mendeleev left gaps in his table for elements, which
he proposed had yet to be discovered.
He was able to predict the properties and
characteristics of the undiscovered elements so
accurately that when they were discovered the
predicted value was quite exact.
The periodic table allowed chemists to organize
and understand their data and predict new
----------------MODERN PERIODIC TABLE-----------------------------
Is organized according to atomic number rather than
atomic mass. (This solved the problems where
different isotopic abundances caused the masses to
be “out of order”. Example: Ar and K, Co and Ni)
The periodic law summarizes the periodic table.
l The Periodic Law: Properties of the chemical elements
recur periodically when the elements are arranged from
lowest to highest atomic numbers
Major Divisions in the Periodic Table
l Period: The set of all elements in a given row going
across the table.
l Group or Family: The set of all elements in a given
column going down the table.
-------------------------------------------------------
THE Family of Periodic table
----Alkali Metal = elements in the first column (Except H)
----Alkaline Earth Metals = The elements in the second column
----Halogens = second column from the end on the right hand side. Starting with Fluorine.
----Noble Gases = Far right side of the table. Starting with helium.
----Lanthanide = elements in the first row shown underneath the table. Starting with lanthanum.
----Actinides = underneath the Lanthanides. Starting with actinium.
..................................................
Metals, Nonmetals, Semiconductors
Metal: reflect light when polished
are opaque
are good conductors of electricity and heat
are malleable or rolled into thin sheets and ductile
are usually solid at room temperatures
..........................................................
Non metals
Non metal: liquids or brittle solids at room temperature
Poor conductors of heat and electricity
Solids are dull to lustrous in appearance and opaque to translucent
Non – Metals can be divided into two subgroups:
1.Very low electrical conductivities
2.Fair to moderate conductivities
.........................................................
Semiconductor
A non-metal having an electrical conductivity, which increases with teperature.
Semiconductors (Metalloids or Semimetals) have properties which resemble metals more than nonmetals
Important difference is that metal conductivity
decreases with increasing temperature whereas the electrical conductivity of semiconductors
increases with increasing temperature.
0.................................................
2011年5月15日星期日
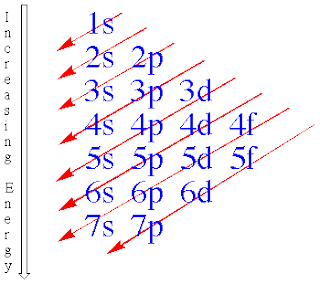
The Electron configuration
-Energy Level is a specific amount of energy which an electron in an atom can possess
- n is the # of energy level
-the energy different between 2 particular energy level is called quantum of energy
-Orbit is the actual region of space occupied by an electron in a particular energy level
- n=1:only the s-type is possible
- n=2:the s and p types are possible
- n=3:the s p and d types are possible
- n=4: the s p d f types are possible
-----------------------------------
- s type sub shell consists of 1 s-orbital
- p type sub shell consists of 3 p-orbital
- d type sub shell consists of 5 d-orbital
- f type sub shell consists of 7 f-orbital
-2 Electron can be placed in each orbit
-a shell is the set of all orbitals having the same N- value
E.g the third shell consists of the 3s 3p 3d orbitals
-a subshell is a set of orbitals of the same type
- An electron configuration is a description of which orbitals in an stom electron configuration
E.g Ne(1s2 2s2 2p6)
=---------------------------------------------
Core Notation
-the set of electrons belonging to a given atom can be divided into 2 subsets: the core electrons and th outer electrons
-CORE: the set of electrons with the configuration of the nearest noble gas having an atomic number Less than that of the atom being considered
E.g Al(1s2 2s2 2p6 3s2 3p1)------>Al([Ne] 3s2 3p1 )
CORE OUTER
EXCEPTION:
Cr([Ar]4s2 3d4)---->Cr([Ar]4s1 3d5) --to make 2 half filled sub shells
Cu([Ar]4s2 3d9)---->Cr([Ar]4s1 3d10)--to make a hall filled sub shell and a filled sub shell
-----------------------------------------------------
- n is the # of energy level
-the energy different between 2 particular energy level is called quantum of energy
-Orbit is the actual region of space occupied by an electron in a particular energy level
- n=1:only the s-type is possible
- n=2:the s and p types are possible
- n=3:the s p and d types are possible
- n=4: the s p d f types are possible
-----------------------------------
- s type sub shell consists of 1 s-orbital
- p type sub shell consists of 3 p-orbital
- d type sub shell consists of 5 d-orbital
- f type sub shell consists of 7 f-orbital
-2 Electron can be placed in each orbit
-a shell is the set of all orbitals having the same N- value
E.g the third shell consists of the 3s 3p 3d orbitals
-a subshell is a set of orbitals of the same type
- An electron configuration is a description of which orbitals in an stom electron configuration
E.g Ne(1s2 2s2 2p6)
=---------------------------------------------
Core Notation
-the set of electrons belonging to a given atom can be divided into 2 subsets: the core electrons and th outer electrons
-CORE: the set of electrons with the configuration of the nearest noble gas having an atomic number Less than that of the atom being considered
E.g Al(1s2 2s2 2p6 3s2 3p1)------>Al([Ne] 3s2 3p1 )
CORE OUTER
EXCEPTION:
Cr([Ar]4s2 3d4)---->Cr([Ar]4s1 3d5) --to make 2 half filled sub shells
Cu([Ar]4s2 3d9)---->Cr([Ar]4s1 3d10)--to make a hall filled sub shell and a filled sub shell
-----------------------------------------------------
Atomic structure
-the chemical elements are different from the other by the # of proton
E.g- N:7 proton in the nucleus
- O:8 proton in the nucleus
- Cl: 17 proton in the nucleus
-Atomic Number: the number of protons in the nucleus
---A neutral atom has no charge, # of electrons = # of protons
E.g: The atomic number of Cl is 17
-any atom of Cl has 17 protons
-a neutral Cl has 17 electrons
-electrons are gained or lost roam neutral atom is called Ion
---------------------------------------------
Atomic Mass is the total number of protons ans neutrons
∴ #of protons + #of neutrons = Atomic Mass
E.g Carbon 12/6 C
#of protons = atomic number = 6
#of electrons = 6
#of neutrons = 12-6=6
------------------------------------------------
Isotopes are atomic species having the same atomic number but different atomic masses
E.g 19/9 F + 1/0n ----20/9 F
-----------------------------------------
E.g- N:7 proton in the nucleus
- O:8 proton in the nucleus
- Cl: 17 proton in the nucleus
-Atomic Number: the number of protons in the nucleus
---A neutral atom has no charge, # of electrons = # of protons
E.g: The atomic number of Cl is 17
-any atom of Cl has 17 protons
-a neutral Cl has 17 electrons
-electrons are gained or lost roam neutral atom is called Ion
---------------------------------------------
Atomic Mass is the total number of protons ans neutrons
∴ #of protons + #of neutrons = Atomic Mass
E.g Carbon 12/6 C
#of protons = atomic number = 6
#of electrons = 6
#of neutrons = 12-6=6
------------------------------------------------
Isotopes are atomic species having the same atomic number but different atomic masses
E.g 19/9 F + 1/0n ----20/9 F
-----------------------------------------
2011年4月11日星期一
Percent Yield & Percent Purity
A: Percent Yield
-A percent yield in chemical equations is used because chemical reactions generally
do not produce the predicted amount of a substance predicted by chemists.
The percent yield is generally determined by the masses that are used in a chemical reaction as
well as by determining the mole ratio
>Ratio of amount of product obtained to amount of product expected by calculation, expressed as %
________________________________
%Yield = |grams of actual product recovered | X 100%
|grams of product expected from stoichi |
--------------------------------------------------------------------------------------------------------------------------------------
Example: If you burn 12 grams of carbon to make CO2, then amount of carbon dioxide expected is one mol of CO2 or 44 grams of CO2. Sadly the amount you will get will probably be less than 44 grams and more like 34 grams of CO2. The problem is a competing reaction that happens. Some carbon reacts to make CO.
The amount of carbon dioxide produced, 34 grams of CO2 is only 77% and not 100 % of the expected 44 grams.
---------------------------------------------------------------------------------------------------------------------------------
B: Percent Purity
-Calculate how much reactant that actually is available to react
-----------------------------------------------------------------------------------------------
-A percent yield in chemical equations is used because chemical reactions generally
do not produce the predicted amount of a substance predicted by chemists.
The percent yield is generally determined by the masses that are used in a chemical reaction as
well as by determining the mole ratio
>Ratio of amount of product obtained to amount of product expected by calculation, expressed as %
________________________________
%Yield = |grams of actual product recovered | X 100%
|grams of product expected from stoichi |
--------------------------------------------------------------------------------------------------------------------------------------
Example: If you burn 12 grams of carbon to make CO2, then amount of carbon dioxide expected is one mol of CO2 or 44 grams of CO2. Sadly the amount you will get will probably be less than 44 grams and more like 34 grams of CO2. The problem is a competing reaction that happens. Some carbon reacts to make CO.
2 C(s) + O2(g) --- > 2 CO(g)
The carbon participating in this "side" reaction will not be able to make CO2. The reaction will not yield 100% of the expected CO2.The amount of carbon dioxide produced, 34 grams of CO2 is only 77% and not 100 % of the expected 44 grams.
---------------------------------------------------------------------------------------------------------------------------------
B: Percent Purity
-Calculate how much reactant that actually is available to react
%Purity = Mass of Pure Substance =x 100%
Mass of Impure sample
2011年4月7日星期四
Excess and Limiting
A: Excess Quantity
-A balanced equation describes what should happen in a chemical reaction
Sometimes necessary to add more of one reactant than the equation
-One reactant is the Excess quantity and some of it will be left over,the second of reactant is used up to -----limiting Quantity
----------------------------------------------------------------------------------------
Example:
How many grams of OCl2 will be formed when 44.0g of O2 react with 97.0g of Fl2?
Step1: balance the equation
2Cl2+O2--->2OCl2
Step2: convert both reactants to the desired product
44g mol O2 x 2molOCl2 x 87g = 239g OCl2
32g 1mol O2 1mol OCl2
97.0gmol Cl2 x 2mol OCl2 x 87g =119gOCl2
71g 2Cl2 1mol OCl2
According to the answer, Cl2 is limiting reactant and O2 is excess reactant
------------------------------------------------------------------------------------------------------------------
-A balanced equation describes what should happen in a chemical reaction
Sometimes necessary to add more of one reactant than the equation
-One reactant is the Excess quantity and some of it will be left over,the second of reactant is used up to -----limiting Quantity
----------------------------------------------------------------------------------------
Example:
How many grams of OCl2 will be formed when 44.0g of O2 react with 97.0g of Fl2?
Step1: balance the equation
2Cl2+O2--->2OCl2
Step2: convert both reactants to the desired product
44g mol O2 x 2molOCl2 x 87g = 239g OCl2
32g 1mol O2 1mol OCl2
97.0gmol Cl2 x 2mol OCl2 x 87g =119gOCl2
71g 2Cl2 1mol OCl2
According to the answer, Cl2 is limiting reactant and O2 is excess reactant
------------------------------------------------------------------------------------------------------------------
Stoichiometry-Calculation involving Particle-moles-Mass
E.g
Zn+2HCl---->H2+ZnCl2
1how many grams of Zn are required to produce 6mole of hydrogen
6molH2 x 1mol Zn x 65.4g =392g Zn
1mol H2 1mole Zn
----------------------------------------------------------------------------------------------------------------------
C3H8+5O2---->3CO2+4H2O
a) What mass of CO2 is produced by reacting 3.00mole of O2?
3.00mol O2 x 3mol CO2 x 44.0g CO2 =79.2gCO2
5mol O2 1mol CO2
b)What mass of C3H8 is required to produce 100.0g of H2O?
100.0gH2O x 1mol H2O x 1mol C3H8 x 44.0gC3H8 =61.1g
18.0gH2O 4mol H2O 1mol C3H8
c) If a sample o propane is burned what mass of h2o is produced if the reaction also produces 50.0L of CO2 at STP?
50.0L CO2 x 1mol CO2 x 4mol H2O x 18.0g H2O = 53.6g
22.4LCO2 3molCO2 1mol H2O
--------------------------------------------------------------------------------------------------------------------
Zn+2HCl---->H2+ZnCl2
1how many grams of Zn are required to produce 6mole of hydrogen
6molH2 x 1mol Zn x 65.4g =392g Zn
1mol H2 1mole Zn
----------------------------------------------------------------------------------------------------------------------
C3H8+5O2---->3CO2+4H2O
a) What mass of CO2 is produced by reacting 3.00mole of O2?
3.00mol O2 x 3mol CO2 x 44.0g CO2 =79.2gCO2
5mol O2 1mol CO2
b)What mass of C3H8 is required to produce 100.0g of H2O?
100.0gH2O x 1mol H2O x 1mol C3H8 x 44.0gC3H8 =61.1g
18.0gH2O 4mol H2O 1mol C3H8
c) If a sample o propane is burned what mass of h2o is produced if the reaction also produces 50.0L of CO2 at STP?
50.0L CO2 x 1mol CO2 x 4mol H2O x 18.0g H2O = 53.6g
22.4LCO2 3molCO2 1mol H2O
--------------------------------------------------------------------------------------------------------------------
CH6:Stoichiometry-Calculation involving Reaction
-Stoichio: element
Metry: measurement
-It's the study of calculating the amount of reactant used in a chemical reaction and how many product produced by the reaction
-----------------------------------------------------------------------------------
4NH3+5O2---->6H2O+4NO
4:5:6:4--the mole ratio
-Balanced chemical equation are required
*Coefficient in balanced equation tell us the number of moles reacted or produced and also be used a conversion factors
-N2+3H2---->2NH3
N2:H2---1:3
NH3:H2---2:3
-------------------------------------------------------------------------------
Example:
CH4+2O2---CO2+2H2O
-0.15molCH4 x 1molCO2 =0.15molCO2
1molCH4
--------------------------------------------------------------------------------------
2011年2月24日星期四
Endothermic and Exothermic Reaction
A: -Endothermic: absorb energy from surrounding
-Exothermic: release energy to surrounding
Ex: Instant ice pack absorb energy and are Endothermic reaction
Explosions release energy are exothermic reaction
-Molecules are held together by chemical bonds
-Add energy to break bonds---->Endothermic
-Give off energy to form bonds--->Exothermic
*Enthalpy, "H" is the heat contained in the system
B: We can chart the potential energy of the chemicals as they change from reactants to products
-Reactants start with a certain amount of energy, energy is added to start the reaction and then energy is released as the reaction processes
-Energy of reactant: total potential energy of all reactants in the reactions
-Energy of product: total potential energy of all products in the reactions
-Energy of activated complex: potential energy of the transition state between reactant-product
-Activation energy: the energy that must be added to get the reaction to progress
-Delta H: the change in potential energy during the reaction
C: The energy in Equation
Ex: CH4+2O2--->CO2+2H2O+812KJ
higher lower
energy energy
-------------------------------------------------------------------------------------
-Exothermic: release energy to surrounding
Ex: Instant ice pack absorb energy and are Endothermic reaction
Explosions release energy are exothermic reaction
-Molecules are held together by chemical bonds
-Add energy to break bonds---->Endothermic
-Give off energy to form bonds--->Exothermic
*Enthalpy, "H" is the heat contained in the system
B: We can chart the potential energy of the chemicals as they change from reactants to products
-Reactants start with a certain amount of energy, energy is added to start the reaction and then energy is released as the reaction processes
-Energy of reactant: total potential energy of all reactants in the reactions
-Energy of product: total potential energy of all products in the reactions
-Energy of activated complex: potential energy of the transition state between reactant-product
-Activation energy: the energy that must be added to get the reaction to progress
-Delta H: the change in potential energy during the reaction
C: The energy in Equation
Ex: CH4+2O2--->CO2+2H2O+812KJ
higher lower
energy energy
-------------------------------------------------------------------------------------
Types of Reactions
-6 generalized types of reaction
-Synthesis
-Decomposition
-Single/Double Replacement
-Combustion
-Neutralization
-----------------------------------------------------------------------------------------------
A: Synthesis A+B --> C
-combines two or more reactants to form one product
Ex: K + Cl --> KCl
------------------------------------------------------------------------------------------
B: Decomposition C--> A+B
-one compound breaks down to two or more reactants
Ex: CaCO3 --> CaO+CO2
---------------------------------------------------------------------------------------------
C: Single Replacement A+BC --> AC+B(A=metal) or A+BC--> AB+C(A=non metal)
-one element is replaced by another in a compound
Ex: 2Al+3FeCl2--> 2Fe+AlCl3
Cl2+2KBr-->Br2+2KCl
*some metals are more reactive than other metal, same as non- metal
*using the Activity Series ----an element higher up on the series replaces the ion below it on the table
Ex: 2Li+MgCl2-->2LiCl+Mg (yes)---Li is higher than Mg on the table
----------------------------------------------------------------------------------------------
D: Double Replacement AB+CD-->AD+BC
-a double replacement is a reaction between two ionic compounds usually in solution
Ex: Na2CO3(aq)+CaCl2(aq)-->CaCO3(s)+2NaCl(aq)
*According to the Solubility Table, if there is a Solid in the product side;
this equation would be reacted
Net Ionic Equation: Ca2+ + Cl1- -->CaCl2
---------------------------------------------------------------------------------------------
E: Combustion AB+O2----->AO+BO
-A combustion reaction is a reaction reactants are chemical to be burned
and the oxygen that it reacts with.
Ex:C4H8+6O2--->4CO2+4H2O
----------------------------------------------------------------------------------------------
F: Neutralization HA+ BOH--->H2O+AB
-A Neutralization reaction is a special double replacement reaction where acids react withe bases to produce water and an ionic salt as products
Ex: 2HBr(aq)+Sr(OH)2(aq)--->SrBr2(aq)+H2O(l)
---------------------------------------------------------------------------
-Synthesis
-Decomposition
-Single/Double Replacement
-Combustion
-Neutralization
-----------------------------------------------------------------------------------------------
A: Synthesis A+B --> C
-combines two or more reactants to form one product
Ex: K + Cl --> KCl
------------------------------------------------------------------------------------------
B: Decomposition C--> A+B
-one compound breaks down to two or more reactants
Ex: CaCO3 --> CaO+CO2
---------------------------------------------------------------------------------------------
C: Single Replacement A+BC --> AC+B(A=metal) or A+BC--> AB+C(A=non metal)
-one element is replaced by another in a compound
Ex: 2Al+3FeCl2--> 2Fe+AlCl3
Cl2+2KBr-->Br2+2KCl
*some metals are more reactive than other metal, same as non- metal
*using the Activity Series ----an element higher up on the series replaces the ion below it on the table
Ex: 2Li+MgCl2-->2LiCl+Mg (yes)---Li is higher than Mg on the table
----------------------------------------------------------------------------------------------
D: Double Replacement AB+CD-->AD+BC
-a double replacement is a reaction between two ionic compounds usually in solution
Ex: Na2CO3(aq)+CaCl2(aq)-->CaCO3(s)+2NaCl(aq)
*According to the Solubility Table, if there is a Solid in the product side;
this equation would be reacted
Net Ionic Equation: Ca2+ + Cl1- -->CaCl2
---------------------------------------------------------------------------------------------
E: Combustion AB+O2----->AO+BO
-A combustion reaction is a reaction reactants are chemical to be burned
and the oxygen that it reacts with.
Ex:C4H8+6O2--->4CO2+4H2O
----------------------------------------------------------------------------------------------
F: Neutralization HA+ BOH--->H2O+AB
-A Neutralization reaction is a special double replacement reaction where acids react withe bases to produce water and an ionic salt as products
Ex: 2HBr(aq)+Sr(OH)2(aq)--->SrBr2(aq)+H2O(l)
---------------------------------------------------------------------------
2011年2月22日星期二
Balancing Equation
-The aim of balancing an equation is to make the number of atoms of each side is equal
STEP:
1.balance the atoms which only occur in one molecule
2.balance whole groups whenever possible (SO4,NO3,ClO3...)
3.Balance atoms which occur in elemental form last
1PbO2+4HBr--->2H2O+1PbBr2+1Br2
---------------------------------------------------------------------------
STEP:
1.balance the atoms which only occur in one molecule
2.balance whole groups whenever possible (SO4,NO3,ClO3...)
3.Balance atoms which occur in elemental form last
1PbO2+4HBr--->2H2O+1PbBr2+1Br2
---------------------------------------------------------------------------
2011年2月21日星期一
Translating Word Equation
1: Calcium carbonate solid and hydrobromic acid react to form carbon dioxide gas, aqueous calcium bromide and liquid water
--CaCO3(s)+HBr(aq)---->CO2(g)+CaBr2(aq)+H2O
2: Aqueous sodium hydrogen carbonate reacts with sulphuric acid to produce aqueous sodium sulphate water, and carbon dioxide gas.
--2NaHCO3(s)+H2SO4(aq)---->Na2SO4(aq)+2H2O(l)+2CO2(g)
3: Sodium metal reacts with water to produce solid sodium hydroxide and hydrogen gas
--2Na+2H2SO4(aq)---->2NaOH(aq)+H2(g)
--CaCO3(s)+HBr(aq)---->CO2(g)+CaBr2(aq)+H2O
2: Aqueous sodium hydrogen carbonate reacts with sulphuric acid to produce aqueous sodium sulphate water, and carbon dioxide gas.
--2NaHCO3(s)+H2SO4(aq)---->Na2SO4(aq)+2H2O(l)+2CO2(g)
3: Sodium metal reacts with water to produce solid sodium hydroxide and hydrogen gas
--2Na+2H2SO4(aq)---->2NaOH(aq)+H2(g)
2011年1月16日星期日
diluting solutions to prepare workable solution
-chemicals are shipped around the world in their most concentrated forms
-need to be able to make solutions of any concentration from a more concentrated source.
Ex: 2L 16.0 HCl I need 0.800L of 2.00M HCl
the key idea is that the moles of solute is constant (more water in less concentrated solute)
Formula: M1L1=M2L2
16M HCl x L1= 2.00M HCl x 0.800L=1.60moles
-need to be able to make solutions of any concentration from a more concentrated source.
Ex: 2L 16.0 HCl I need 0.800L of 2.00M HCl
the key idea is that the moles of solute is constant (more water in less concentrated solute)
Formula: M1L1=M2L2
16M HCl x L1= 2.00M HCl x 0.800L=1.60moles
2011年1月12日星期三
Molar Concentration or Molarity of solution
-Molar Concentraion
- A homogeneous mixture one substance is dissolved in another---solution
- The chenmical that is in the smaller quantity is called solute
larger quantity is called solvent
- Molar Concentration or Molarity is the number of moles of solute in one L of a lolution, we use M to denote molar concentration and it has the units of "mole/L"
FOMULA: - Molarity = moles of solute(mol)/Volume of soution(L) or M = mol/L
Ex: Calculate the molar concentration of a soluion that has 0.510 moles of NaOH in 1.400L of solution.
0.510mol/1.400L= 0.364M or 0.364mol/L
Ex: Calculate the number of grams of calciumhyfroxide in 1.30L of a 0.75M Ca(OH)2 solution.
moles Ca(OH)2 = 0.75M x 1.30L = 0.975mol
0.975mol x 74.1 = 72g
- A homogeneous mixture one substance is dissolved in another---solution
- The chenmical that is in the smaller quantity is called solute
larger quantity is called solvent
- Molar Concentration or Molarity is the number of moles of solute in one L of a lolution, we use M to denote molar concentration and it has the units of "mole/L"
FOMULA: - Molarity = moles of solute(mol)/Volume of soution(L) or M = mol/L
Ex: Calculate the molar concentration of a soluion that has 0.510 moles of NaOH in 1.400L of solution.
0.510mol/1.400L= 0.364M or 0.364mol/L
Ex: Calculate the number of grams of calciumhyfroxide in 1.30L of a 0.75M Ca(OH)2 solution.
moles Ca(OH)2 = 0.75M x 1.30L = 0.975mol
0.975mol x 74.1 = 72g
2011年1月9日星期日
Percent composition
- percentage by mass of a "species" in a chemical formula
Ex: what percentage composition of CO2
*assume you have one mole
CO2 = 44g/mole
C = 12g/mole----12g/mole/(44g/mole) x 100%=27.3%
O = 32g/mole-----32g/mole/(44g/mole) x 100%=72.3%
*percent composition of a compound tells you which elements are in the compound how much of each here is
Ex: calculate % composition of
-Cu(NO3)2
Cu(NO3)2 = 63.5g+28g+96g=187.5g/mole
Cu 63.5g/ 187.5g=33.9%
N 28g/187.5g=14.9%
O 96g/187.5g=51.2%
Ex: what percentage composition of CO2
*assume you have one mole
CO2 = 44g/mole
C = 12g/mole----12g/mole/(44g/mole) x 100%=27.3%
O = 32g/mole-----32g/mole/(44g/mole) x 100%=72.3%
*percent composition of a compound tells you which elements are in the compound how much of each here is
Ex: calculate % composition of
-Cu(NO3)2
Cu(NO3)2 = 63.5g+28g+96g=187.5g/mole
Cu 63.5g/ 187.5g=33.9%
N 28g/187.5g=14.9%
O 96g/187.5g=51.2%
Empirical+Molecular Formula
-Empirical formula --gives the largest term ratio of atoms (or moles) in the formula
*all ionic compounds are empirical formula
Ex: C4H10---molecular formula
C2H5----Empirical formula
Ex: -consider that we have 10.87g of Fe and 4.66g of O, what is the empirical formula?
MM of Fe---55.8g----- 10.87g x (1mole/55.8g) =0.195mol
MM of O------16g---------4.66 x (1mole/16g) = 0.291mol
-divide both by 0.195(the smallest molar amount)
Fe 1(0.195/0.195) x2 O 1.5(0.291/0.195)x2
Fe 2 O 3
- scale ratio to whole number----Fe2O3
Ex: A compound contains 31.9%K , 28.9%Cl2, 39.2%O, what is the empirical formula
*assume you have a hundred gram
K--31.9g x (1mole/39.1g)=0.816mole/0.814-------------1
Cl2--28.9g x (1mole/ 35.5g)=0.814mole/0.814----------1
O--39.2g x (1mole/16g)=2.45mole/0.814-----------------3
*KClO3---empirical formula
____________________________________________________________________________________
-Molecular formula: is a multiple of the empirical formula and shows the actual number of atoms that combine to form a molecule.
**To multiple
N= molar mass of the compound/molar mass of the empirical formula**
Ex : A molecule has an empirical formula of C2H5 and a molar mass of 58g /mole, what is the molecular formula?
MM of C2H5= 29g/mole
N= (58g/mole)/(29g/mole)=2
2 x C2H5 = C4H10---molecular formula
_________________________________________________________________
*all ionic compounds are empirical formula
Ex: C4H10---molecular formula
C2H5----Empirical formula
Ex: -consider that we have 10.87g of Fe and 4.66g of O, what is the empirical formula?
MM of Fe---55.8g----- 10.87g x (1mole/55.8g) =0.195mol
MM of O------16g---------4.66 x (1mole/16g) = 0.291mol
-divide both by 0.195(the smallest molar amount)
Fe 1(0.195/0.195) x2 O 1.5(0.291/0.195)x2
Fe 2 O 3
- scale ratio to whole number----Fe2O3
Ex: A compound contains 31.9%K , 28.9%Cl2, 39.2%O, what is the empirical formula
*assume you have a hundred gram
K--31.9g x (1mole/39.1g)=0.816mole/0.814-------------1
Cl2--28.9g x (1mole/ 35.5g)=0.814mole/0.814----------1
O--39.2g x (1mole/16g)=2.45mole/0.814-----------------3
*KClO3---empirical formula
____________________________________________________________________________________
-Molecular formula: is a multiple of the empirical formula and shows the actual number of atoms that combine to form a molecule.
**To multiple
N= molar mass of the compound/molar mass of the empirical formula**
Ex : A molecule has an empirical formula of C2H5 and a molar mass of 58g /mole, what is the molecular formula?
MM of C2H5= 29g/mole
N= (58g/mole)/(29g/mole)=2
2 x C2H5 = C4H10---molecular formula
_________________________________________________________________
订阅:
博文 (Atom)


 =delta
=delta